mito functional nutrition | Funktionelle Getränke
Your daily refreshment
Mit der Marke mito functional nutrition decken wir neben unseren Nahrungsergänzungsmitteln, auch funktionelle Ernährung ab. Aufgrund unseres heutigen Lebensstils, Stress und Zeitmangel, fällt es uns im Alltag oft nicht leicht, unseren Körper optimal zu versorgen. Unser functional** drink kann eine einfache Alternative bieten, um mehr Vitalität und zelluläre Leistungsfähigkeit 1,2 in deinen Alltag zu integrieren.
1 Eine Dose mito drink enthält die Vitamine Vitamin C, Thiamin, Vitamin B12, Biotin und Pantothensäure. Diese tragen zu einem normalen Energiestoffwechsel bei.**
2 Eine Dose mito drink enthält Pantothensäure. Dies trägt zu einer normalen geistigen Leistung bei.
mito drink Essenz | effektiv & synergetische Inhaltsstoffe
Komplexmischungen wie in der Natur
Tropenfrüchte wie Açaí, Aronia und Mangosteen treffen auf Grüntee und eine erfrischend-perlige Ingwer-Note. Die komplexe Vitaminmischung wird durch Coenzym Q10 und L-Carnitin funktionell ergänzt. Die hochwertigen Inhaltsstoffe sind speziell aufeinander abgestimmt und versorgen dich effektiv und synergetisch mit Mikronährstoffen.

Erfrischt deine Mitochondrien1,2
90% deiner Energie durch gesunde Mitochondrien
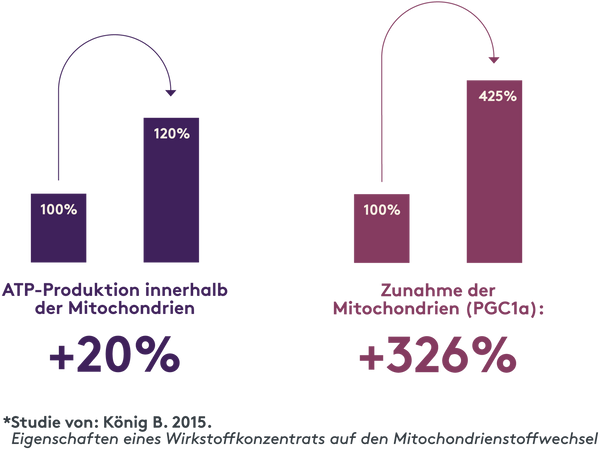
Mitochondrien erzeugen die Energie, die deine Zellen, Organe und dein Gewebe benötigen, um effektiv zu arbeiten. Am Tag produzieren sie im menschlichen Körper rund 40 kg Energie in Form von ATP. Die aktivierende Wirkung des Getränkes auf die Mitochondrien wurde durch eine Studie in einem unabhängigen Labor* bestätigt.
*Die Studie findest du hier.
1 Eine Dose mito drink enthält die Vitamine Vitamin C, Thiamin, Vitamin B12, Biotin und Pantothensäure. Diese tragen zu einem normalen Energiestoffwechsel bei.
2 Eine Dose mito drink enthält Pantothensäure. Dies trägt zu einer normalen geistigen Leistung bei.
Nachhaltigkeit ist uns wichtig
Wir prüfen jeden unserer Schritte in Bezug auf unseren ökologischen Fußabdruck
und setzen dabei auf innovative Maßnahmen.
FAQs zu mito functional nutrition
Wie können wir dir weiterhelfen?
Du hast Fragen zum mito drink, der Produktion oder unserer Philosophie?
Schau dich gerne in unserem FAQ Bereich um.
Ist der mito drink ein Energydrink?
Nicht im klassischen Sinne – er ist ein functional** drink für deine Zellen, der dich mit wichtigen Vitaminen und Mineralien versorgen kann. Natürliche Zutaten wie Grüntee und Guarana enthalten von Natur aus Koffein. Unsere empfohlene Tagesdosis liegt bei einer Dose. Der mito drink entfaltet seine aktivierende (1) Wirkung zu jeder Tageszeit. Wir empfehlen das Getränk vor dem Sport, während der Mittagspause oder in der Arbeit zu trinken.
1. Trägt zu einem normalen Energiestoffwechsel bei (Vitamin C, Thiamin, Riboflavin, Niacin, Vitamin B6, Vitamin B12, Biotin, Pantothensäure, Calcium, Magnesium).**
Ist der mito drink für meine Ernährung geeignet?
Unser mito drink ist reich an Vitaminen und Mineralien und ist somit eine tolle Ergänzung zu deiner Ernährung – und das ganz ohne Pillen schlucken. Eine Dose enthält dabei lediglich 3g Süße inform von Agavendicksaft und Stevia. Zudem ist unser drink vegan.
Ist der mito drink vegan und biologisch?
Unser mito drink ist vegan und gleichzeitig auch frei von den bekanntesten 14 Allergenen gemäß Verordnung (EU) Nr. 1169 / 2011, Anhang II. Zu diesen gehören: Gluten, Krebstiere, Ei, Fische, Erdnüsse, Sojabohnen, Milch, Schalenfrüchte e.g. Mandeln, Haselnüsse, Walnüsse etc., Sellerie, Senf, Sesamsamen, Schwefeldioxid und Sulphite von mehr als 10mg/kg, Lupinen und Weichtiere.
Kann das Aluminium der Dosen in den Inhalt des Getränks übergehen?
Auf keinen Fall. Wir verwenden BPA freie Dosen die innen beschichtet sind, sodass der Inhalt nicht in Kontakt mit dem Metall kommt. Die Dose schützt den Inhalt optimal vor Lichteinflüssen und möglichen Keimen.
Kann ich den mito drink zusammen mit MITOcare Produkten einnehmen?
Der mito drink kann ohne Probleme mit Produkten von MITOcare kombiniert werden. Bitte beachte hierbei, dass hierdurch keine Mahlzeiten, oder generell eine gesunde Ernährung ersetzt werden soll.
Muss ich den mito drink kühlen?
Der mito drink ist nicht kühlpflichtig. Um dir aber den absoluten Frischekick zu geben, empfehlen wir, den mito drink vor dem Verzehr kalt zu stellen.
Warum ist der mito drink genau das richtige Getränk für dich?
Der mito drink ist kein gewöhnliches Getränk. Basierend auf wissenschaftlichen Studien, wurde von Ärzten eine reichhaltige, komplexe und ganzheitliche Rezeptur entwickelt, um deinem Körper mit essentiellen Vitaminen und Mineralien zu versorgen. Du kannst mit einer Dose mito drink deinen Tagesbedarf an wichtigen Mikronährstoffen decken.
Warum sind Dosen wirklich nachhaltig?
Unsere Dose besteht zu 100% aus recycelbarem Aluminium. Bei der Aufbereitung der Dosen wird deutlich weniger Energie als die Aufbereitung von Altglas benötigt. Leichtes und dünnes Material bedeutet effizienter Transport und geringeres Gewicht und damit weniger Treibhausgase und eine sehr positive Umweltbilanz. Schnelles Kühlen ist ganz einfach möglich – alle anderen Materialien brauchen tatsächlich viel länger. Das bedeutet deutlich weniger Stromverbrauch. Der Recycling-Prozess ist ohne Substanz- und Qualitätsverlust – eben ganz anders als bei PET.
Warum verwenden wir keine PET Flaschen?
Dosen sind in der Abfüllung viel hygienischer, weil sie bei höheren Temperaturen im Vergleich zu Plastik keimfrei gemacht werden können. Außerdem ist durchsichtiges Material ungünstig für lichtempfindliche Vitamine. Besonders die Vitamine B6, B12, Vitamin C und E sind sehr lichtempfindlich.
Was bedeutet der Begriff mito?
mito ist die Kurzform für Mitochondrien - die Kraftwerke deiner Zellen. Warum du die Funktion der Mitochondrien kennen solltest – und wie diese durch unseren mito drink unterstützt werden können – erfährst du in unserem Wissensblog.
Was ist die mito drink Essenz?
Die mito drink Essenz ist eine mit Ärzten entwickelte, hochsensible Komplexmischung verschiedener Mikronährstoffe.
Was sind Mitochondrien?
Mitochondrien sind die Kraftwerke deiner Zellen. 90 Prozent der Energie deines Körpers kommt von deinen Mitochondrien. In jeder Zelle befinden sich 2000 bis 12000 Mitochondrien als bohnenförmige Zell-Organellen. Sie erzeugen die Energie, die deine Zellen, Organe und dein Gewebe benötigen, um effektiv zu arbeiten. Am Tag produzieren sie in deinem Körper rund 40 kg Energie in Form von ATP. Mehr dazu findest du in unserem Blogbeitrag.
Wo wird der mito drink hergestellt?
Der mito drink wird in Deutschland produziert und abgefüllt.
Wonach schmeckt der mito drink?
Für den einzigartigen Geschmack des mito drinks verwenden wir nicht nur Extrakte aus Tropfenfrüchten sondern auch Gemüse- und Kräuterkonzentrate in unserer Rezeptur. Dadurch schmeckt der Drink weder zu süß noch zu fruchtig – ganz besonders eben. Diese Kombination trifft auf ein leichtes Prickeln von Ingwer und rundet so das Geschmackserlebnis ab
Welche Aromen verwenden wir?
Wir verwenden in unserer Rezeptur Aroma Koffein, das zugesetzte Koffein ist natürlichen Ursprungs. Außerdem enthält der mito drink natürliches Ingwer Aroma.

Kontaktformular
Du hast Interesse an einer Kooperation mit mito functional nutrition oder möchtest den mito drink gerne als Drittverkäufer in dein Sortiment aufnehmen? Schicke uns deine Anfrage über das Kontaktformular oder an info@mito-drink.com. Wir werden uns schnellstmöglich bei dir melden. Alternativ kannst du uns unter 089 2488163 380 telefonisch erreichen.




















